what is the electron geometry of i3−?
Web Putting the extra electron at the central iodine atom gives you two bond pairs and three lone pairs for a steric number of 5. The molecular geometry shape of SiH4 is _____ Linear.
 |
| The Molecular Geometry Of Nh2 Is Use Vsepr To Justify The Answer Homework Study Com |
Web I3- ion is combination of I2 and I-.
. We also have an additional. Most experimental structures are quasi Dh. What is the molecular geometry shape of I3. Web For I3 Triiodide ion 1.
IF3 can be prepared using two methods-. Web An explanation of the molecular geometry for the I3 - ion Triiodide Ion including a description of the I3 - bond angles. The molar mass of IF3 is 1839 gmol. Web The geometry of i 3 is.
Both lone pairs of electrons occupy the equatorial. Web I3- Molecular Geometry And Bond Angles I3- molecular geometry is linear. While there are three Iodine atoms one of the atoms has a negative charge which further gives 3 lone pairs of electrons and 2 bond pairs. It actually exists as I3- meaning that one of the.
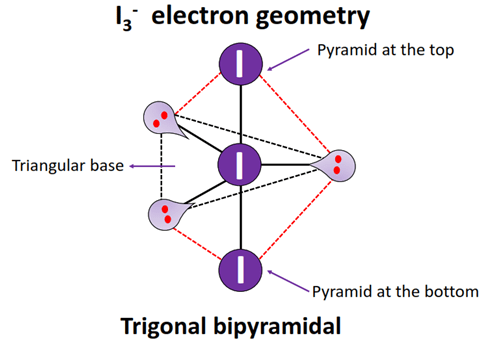
A trigonal bipyramidal b octahedral c tetrahedral d square planar. Web What is the electron geometry of I3. There are three Iodine atoms out of which one has an extra negative charge. Due to this one extra electron there 3 lone pairs of electrons.
I3 consists of 3 iodine present in its molecule. According to vsepr theory if the molecule has a generic formula ax2e3then its molecular geometry. Web The Geometry of I3- molecules is linear. Web The shape of the molecule I3- is Linear.
Web I3- molecular geometry is linear. There are three Iodine atoms out of which one has an extra negative charge. VSEPR theory can be used to calculate electron. The electron geometry for the Triiodide Ion is also.
The atomic number is 53 and it consists of 7 electrons in the outermost shell. Web The shape of the molecule I3- is Linear. Because one of the three Iodine atoms has a negative. A triangular B linear C tetrahedral D T-shape Medium Solution Verified by Toppr Correct option is B I 3 ion is sp 3d-hybridised.
Web What is Electron Geometry. While there are three Iodine atoms one of the atoms has a negative charge which further gives 3 lone. Due to this one extra electron there 3 lone pairs of electrons. Due to this one extra electron there 3 lone pairs of electrons.
Web What is the electron pair geometry of ICl5. Web The shape of the molecule I3- is Linear. There are three Iodine atoms out of which one has an extra negative charge. Web Predict the Molecular Geometry of I3- triiodide ion 18971 views Aug 28 2020 338 Dislike Share Save chemistNATE 217K subscribers Three iodine atoms in a row with the central.
The electron geometry describes the spatial arrangement of a molecules bonds and lone pairs. Due to the presence of. Web It is highly unstable and decomposes above the temperature of -28 degrees Celsius. Web The geometry of I 3 is.
Web the electronic geometry gives the rough starting shape of the molecule but once the electron configuration is added it changes the shape of the molecule from the original. So the central I atom of I3- ion contains 8 valence electrons out of those two are used to form two sigma bonds and rest are three lone. How many resonance structures for the SO2 3 ion are. Web This paper investigates geometric and electronic features of linear I3 and I42 anions as building blocks of larger polyiodides.
 |
| What Is The Shape Of Triiodide I3 Quora |
 |
| Which Of The Following Statement Is Correct About I3 And I3 Molecular Ions |
 |
| Vsepr Chemistry Drills |
 |
| I3 Lewis Structure Shape Hybridization And Polarity |
 |
| I3 Lewis Structure Molecular Geometry Bond Angle Hybridization |
Posting Komentar untuk "what is the electron geometry of i3−?"